Effect of Accelerated Aging on Physiological Characteristics of Lentil Seeds
Deepak Rao, Ravish Choudhary, Shiv Kumar Yadav and Sangita Yadav
AgriBio Innovations 2025, 2(1): 13-17
INTRODUCTION
Pulses are a vital group of crops that provide highquality protein, complementing cereal proteins, particularly for India’s predominantly vegetarian population. Despite being the world’s largest producer of pulse crops, India’s pulse production remains significantly lower compared to its cereal production. Pulses have the unique ability to fix atmospheric nitrogen in their root nodules, meeting a significant portion of their nitrogen requirements. Lentil (Lens culinaris Medik.) is a diploid species (2n = 14) (Muehlbauer, 1991) and is a self-pollinating annual plant. It is considered the oldest cultivated legume (Bahl et al., 1993; Rehman et al., 1994) and one of the earliest domesticated grain legumes, dating back to 6000 B.C. Originating in Southwestern Asia, lentils spread from the Near East and Egypt to Central and Southern Europe, the Mediterranean, Ethiopia, Afghanistan, India, Pakistan, China, and later to Latin America (Cubero, 1981; Duke, 1981). The nutritional profile of lentils per 100 g dry weight includes energy 1,477 kJ (353 kcal), carbohydrates 60 g, sugars 2 g, dietary fiber 31 g, fat 1 g, protein 26 g, and significant amounts of vitamins (B1, B2, B3, B5, B6, B9), minerals (calcium, iron, magnesium, phosphorus, potassium, sodium, and zinc), along with water (10.4 g) (Source: USDA Nutrient Database) (Rao et al., 2024). Delouche (1965) first introduced the accelerated aging test for seed quality at the Mississippi State University’s Seed Innovation Research Facility, USA, as a method to evaluate seed viability during storage. Later studies validated the accuracy of this test in predicting the longevity of various seeds under storage conditions (Delouche and Baskin 1973). Seed invigoration (Rao et al., 2023; Rao et al., 2024) refers to post-harvest treatments that enhance seed vigor, improving germination, storability, and field performance compared to untreated seeds (Kalyani et al., 2009). Hormonal seed treatments have become widely used for improving seed germination, seedling growth, and 14 crop yield under adverse conditions (Rhaman et al., 2020). Such treatments can ensure better germination and healthier plants even in challenging environments (Hasanuzzaman and Fotopoulos 2019; Hu et al., 2013). Seed deterioration, characterized by a decline in quality, viability, and vigor, is influenced by factors such as aging, moisture content, and storage temperature (Ellis et al., 1985). Accelerated aging tests, which manipulate moisture and temperature, simulate and speed up the natural seed aging process (Delouche and Baskin 1973). Physiological and biochemical changes during seed aging (Rao et al., 2024) have been widely studied (McDonald, 1999; Jatoi et al., 2004). Seeds often lose viability within days or weeks under storage conditions (Murthy and Kumar 2003). Numerous studies have standardized accelerated aging test procedures for various crops, including Brassica (Bedi et al., 2006), corn (Woltz and Tekrony 2001), chickpea (Roy et al., 1994; Gil et al., 1996), lentil (Fernandez and Johnston 1995), maize (Bako, 2006), mung bean (Murthy and Kumar, 2003), pea (Jatoi et al., 2004), pigeon pea (Kalpana and MadhavRao 1995), and soybean (Delouche and Baskin 1973; Tekrony, 1993). Variability in seed germination, vigor, and storability across chickpea genotypes has been documented, with smaller seeds generally exhibiting superior germination and growth compared to medium or large seeds (Roy et al., 1994; Raje and Khare 1996). The results revealed that the accelerated ageing cause progressive decline in viability of seed (Poojitha et al., 2022). This study aims to analyze the physiological and biochemical changes in lentil seeds during accelerated aging and to identify the most effective treatment among five experimental lentil varieties.
MATERIAL AND METHODS
Experimental Design. The study was conducted to assess the effects of accelerated aging (AA) on seed germination, seedling growth, and vigor indices. Seeds were subjected to controlled aging treatments under specific temperature and duration combinations, and the resulting physiological and morphological parameters were recorded.
Treatments. The treatments included a control (non-aged seeds) and four accelerated aging conditions: AA at 40°C for 8 days (DAS) AA at 42°C for 8 DAS AA at 40°C for 10 DAS AA at 42°C for 10 DAS
Accelerated Aging Protocol. Seeds were placed in sealed containers to maintain high humidity and exposed to the specified temperatures (40°C and 42°C) for the given durations (8 or 10 days). After aging, the seeds were air-dried and tested for germination and vigor.
Germination Test. Germination percentage was determined by placing 100 seeds per treatment in germination trays under standard laboratory conditions. Seeds were observed over 7 days, and the percentage of seeds that produced normal seedlings was recorded.
Seedling Growth Parameters. For each treatment, the following parameters were measured from the germinated seedlings: Shoot Length (cm): Measured from the base to the tip of the shoot. Root Length (cm): Measured from the base to the root tip. Total Seedling Length (TSL, cm): Calculated as the sum of shoot and root lengths. Seedling Vigor Indices (SVI&II)
Statistical Analysis. The data were analyzed using ANOVA to determine the significance of differences between treatments. Critical Difference (CD) values at 𝑃=0.05, P=0.05 were calculated to separate the means.
Equipment Used. Germination trays — Temperature-controlled chamber — Digital caliper for length measurements — Precision balance for seedling dry weight.
RESULTS AND DISCUSSION
Germination Percentage. The germination percentage significantly varied among treatments (Table 1). The control exhibited the highest germination rate (92%), confirming optimal conditions for seed viability. Accelerated aging (AA) at 40°C for 8 days resulted in the lowest germination rate (62%), highlighting the adverse effects of thermal stress and prolonged aging on seed viability. However, AA at 42°C for 8 days showed an improvement in germination (80%), suggesting potential resilience under specific stress conditions. Seeds exposed to 42°C for 10 days demonstrated a recovery in germination percentage (90%), which could indicate the onset of thermal adaptation mechanisms. This trend corroborates earlier findings that moderate levels of stress can sometimes induce physiological adjustments in seeds (Bailly, 2004).
Shoot and Root Length. Shoot and root lengths are critical indicators of seedling vigor and were substantially affected by accelerated aging (Table 1). The control treatment produced the longest shoot (11.62 cm) and root (19.76 cm), reflecting robust seedling development. Under AA at 40°C for 8 days, both shoot (5.05 cm) and root lengths (4.11 cm) drastically decreased, indicating that thermal stress significantly hampers seedling growth. At 42°C for 8 days, shoot and root lengths were moderately improved (11.77 cm and 13.38 cm, respectively). This suggests that while thermal stress affects growth, shorter exposure durations at higher temperatures might mitigate its adverse effects. Similar results have been reported by Basu et al. (2016), who emphasized the importance of stress duration on seedling growth.
Total Seedling Length (TSL). The total seedling length (TSL) was highest in the control group (31.38 cm) (Table 1). TSL was severely reduced in seeds subjected to AA at 40°C for 8 days (6.4 cm) but improved under AA at 42°C for 8 days (25.15 cm). Prolonged aging at 40°C and 42°C for 10 days resulted in intermediate TSL values (15.48 cm and 17.19 cm, respectively). These findings indicate that seedling length is directly proportional to the germination percentage and vigor index.
Seedling Vigor Indices (SVI-I and SVI-II). Seedling vigor indices (SVI-I and SVI-II) reflect the combined effects of germination percentage and seedling growth parameters. In SVI-I, the control treatment outperformed all other treatments (2885), highlighting the superior seed quality under non-stress conditions. AA at 40°C for 8 days led to a dramatic decline in SVI-I (392.72), while AA at 42°C for 8 days exhibited some improvement (200.32), possibly due to shorter aging periods. Prolonged aging at 42°C for 10 days resulted in a higher SVI-I (1580) compared to 40°C for 10 days (1249), suggesting that temperature plays a significant role in determining seed vigor (Table 1 and Figure 1). Similarly, for SVI-II, the control achieved the highest value (2.5872), with the lowest observed at 40°C for 8 days (0.82564). Prolonged aging at 42°C for 10 days resulted in the highest SVI-II (3.59) among the stressed treatments, reflecting better metabolic activity in the seeds (Fig. 2).
Table 1: The effect of AA on different seed quality parameters.
| Treatments | Germination (%) | Shoot Length (cm) | Root Length (cm) | TSL (cm) | SVI-I | SVI-II |
|---|---|---|---|---|---|---|
| Control | 92 | 11.62 | 19.76 | 31.38 | 2885 | 2.5872 |
| AA 40°C for 8 DAS | 62 | 5.05 | 4.11 | 6.4 | 392.72 | 0.82564 |
| AA 42°C for 8 DAS | 80 | 11.77 | 13.38 | 25.15 | 200.32 | 1.116 |
| AA 40°C for 10 DAS | 80 | 5.68 | 9.8 | 15.48 | 1249 | 3.34 |
| AA 42°C for 10 DAS | 90 | 6.5 | 10.69 | 17.19 | 1580 | 3.59 |
| CD (Critical Difference) | 2.5 | 1.2 | 1.5 | 1.4 | 35.5 | 0.58 |
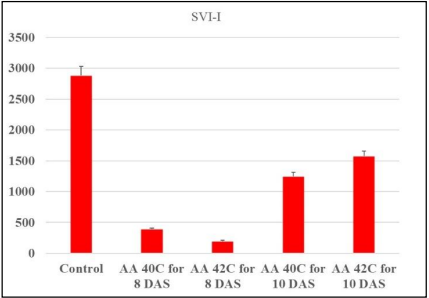
Fig. 1. The effect of the AA on SVI-I after 8 and 10 DAS.
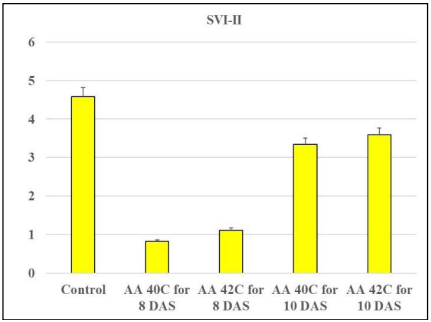
Fig. 2. The effect of the AA on SVI-II after 8 and 10 DAS.
CONCLUSIONS
The findings of this study highlight the significant impact of accelerated aging on lentil seed germination, seedling growth, and vigor indices. The control treatment exhibited optimal results, confirming the importance of maintaining favorable storage conditions for seed viability. Accelerated aging at 40°C for 8 days had the most detrimental effects on germination and seedling growth parameters, emphasizing the adverse influence of prolonged thermal stress. However, treatments at 42°C for 8 days showed improved germination and seedling vigor, suggesting potential mechanisms of thermal resilience and adaptation. These results demonstrate the critical role of stress duration and intensity in determining seed quality and underscore the importance of seed vigor studies for optimizing storage and pre-sowing treatment practices. By identifying superior treatments and understanding seed deterioration processes, this research contributes to enhancing lentil seed quality and ensuring better crop establishment under challenging environmental conditions.
Acknowledgements. The first author expresses gratitude to ICAR-Indian Agricultural Research Institute, New Delhi, India, for generously supporting this study.
REFERENCES
Bailly, C. (2004). Active oxygen species and antioxidants in seed biology. Seed Science Research, 14(2), 93-107.
Bako, C. (2006). Accelerated aging tests for maize seed viability. Seed Science Technology, 34(1), 55-62.
Bahl, J., Jain, S. K. & Raghavan, S. (1993). Lentil production and productivity: Constraints and opportunities. Indian Journal of Pulses Research, 6(1), 1-9.
Basu, A., Poddar, S. & Ray, D. (2016). Effect of temperature stress on seed germination and seedling growth. Indian Journal of Agricultural Sciences, 86(5), 660-665.
Bedi, S., Gill, R. S., & Singh, B. (2006). Effect of aging on seed germination and seedling vigor in Brassica. Seed Research, 34(2), 99- 103.
Cubero, J. I. (1981). Origin, domestication, and evolution of lentils. The Lens Newsletter, 8(1), 3-6.
Delouche, J. C. (1965). Accelerated aging tests for predicting seed longevity. Mississippi State University.
Delouche, J. C. & Baskin, C. C. (1973). Accelerated aging techniques for predicting the relative storability of seed lots. Seed Science Technology, 1(3), 427-452.
Duke, J. A. (1981). Handbook of legumes of world economic importance. New York: Plenum Press.
Ellis, R. H., Hong, T. D. & Roberts, E. H. (1985). Handbook of seed technology for genebanks: Volume II. Rome: International Board for Plant Genetic Resources.
Fernandez, D. & Johnston, M. O. (1995). Studies on seed quality in lentil. Canadian Journal of Plant Science, 75(1), 135-142.
Gil, B., Martínez, C. & Gil, F. (1996). Aging effects on chickpea seeds under storage. Seed Science Research, 6(3), 157-164.
Hasanuzzaman, M., & Fotopoulos, V. (2019). Priming and hormonal treatments for improving seed quality. Frontiers in Plant Science, 10, 876.
Hu, X., Shi, Z. & Lin, H. (2013). Hormonal seed treatments under stress conditions. Journal of Agricultural Science, 151(4), 417-424.
Jatoi, S. M., Afzal, M., Nasim, S. & Siddiqui, S. U. (2004). Seed aging effects on pea germination and vigor. Pakistan Journal of Botany, 36(4), 799-806.
Kalpana, R. & Madhav Rao, R. (1995). Seed storage studies in pigeon pea. Journal of Agricultural Research, 22(3), 50-55.
Kalyani, A., Nair, R. & Thomas, A. (2009). Seed invigoration techniques to enhance crop performance. Journal of Agricultural Science, 78(4), 239-246.
McDonald, M. B. (1999). Seed deterioration: Physiology, repair, and assessment. Seed Science and Technology, 27(1), 177-237.
Muehlbauer, F. J. (1991). Lentil (Lens culinaris Medik.) in the pulse crops of the world. Agronomy Monograph, 28(1), 627-648.
Murthy, U. M. & Kumar, P. (2003). Role of temperature and moisture on seed deterioration in mung bean. Journal of Stored Products Research, 39(1), 35-44.
Poojitha, J., Kumari, Ch. Aruna, Rao, D. Sanjeeva, Rao, P. R. and Siromani, N. (2022). Effect of Accelerated Ageing on Moisture and viability of Rice Genotypes. Biological Forum – An International Journal, 14(3), 625-629.
Raje, R. S. & Khare, D. (1996). Variation in seed germination and vigor in chickpea genotypes. Seed Science and Technology, 24(2), 345-354.
Rao, D., Yadav, S., Choudhary, R., Singh, D., Bhardwaj, R., Barthakur, S. & Yadav, S. K. (2023). Silicic and Humic Acid Priming Improves Micro-and Macronutrient Uptake, Salinity Stress Tolerance, Seed Quality, and Physio-Biochemical Parameters in Lentil (Lens culinaris spp. culinaris). Plants, 12(20), 3539.
Rao, D., Yadav, S., Choudhary, R., Singh, D., Bhardwaj, R., Barthakur, S. & Yadav, S. K. (2024). Unveiling the potential of silicic and humic acid priming in alleviating salinity stress on lentil (Lens culinaris) seed germination in a hydroponic system. Journal of Food Legumes, 37(3), 291-296.
Rao, D., Yadav, S., Choudhary, R. & Singh, D. Integrated Application of Silicic and Humic Acid Seed Priming for Enhanced Germination and Yield of Lentil (Lens culinaris L.). Legume Research-An International Journal, 1, 5.
Rao, D., Rajput, P., Choudhary, R., Yadav, S., Yadav, S. K., Rajput, V. D. & Matić, S. (2024). Multifaceted Characteristics of Biochar and Its Implementation in Environmental Management in a Sustainable Way. Environmental Quality Management, 34(1), e22305.
Rahman, M., Rahman, M. M. & Islam, M. S. (2020). Hormonal priming and seedling vigor under stress conditions. Plant Science Today, 7(3), 187-195.
Rehman, S. Mahmood, T., & Gul, S. (1994). Lentil domestication and spread in the Near East. Lens Newsletter, 21(1), 7-9.
Roy, A., Das, T. K. & Choudhury, S. K. (1994). Seed quality assessment in chickpea. Indian Journal of Agricultural Sciences, 64(6), 379- 382.
Tekrony, D. M. (1993). Seed vigor testing in soybean. Seed Technology, 15(1), 33-39. Woltz, J. M. & Tekrony, D. M. (2001). Accelerated aging tests for corn seed viability. Crop Science, 41(4), 1139-1146.
USDA Nutrient Database. Nutritional composition of lentils per 100 g dry weight. Retrieved from https://fdc.nal.usda.gov/